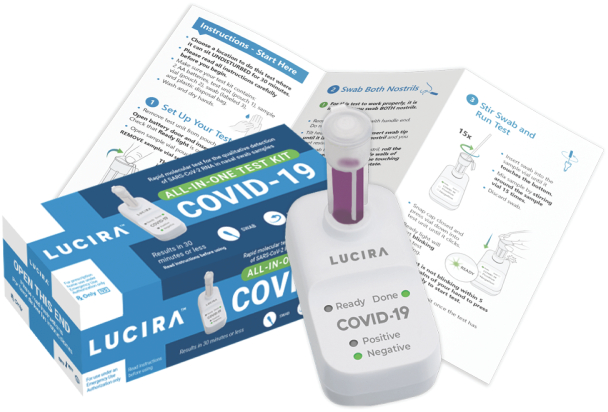
Lucira Health developed the first FDA-authorized home-use molecular diagnostic test for COVID-19.
As Director of Engineering at Lucira Health, Dr. Myers helped build core R&D, manufacturing, and quality functions within the company. He led the hardware and software development of the company’s diagnostic platform from concept through successful commercial launch and manufacturing scale-up. He is co-inventor on seven patent families related to the product.
The Lucira test features highly cost-optimized microfluidics and electronics which perform a multiplexed nucleic amplification assay for rapid diagnostics in an ergonomic, easy-to-use form factor.