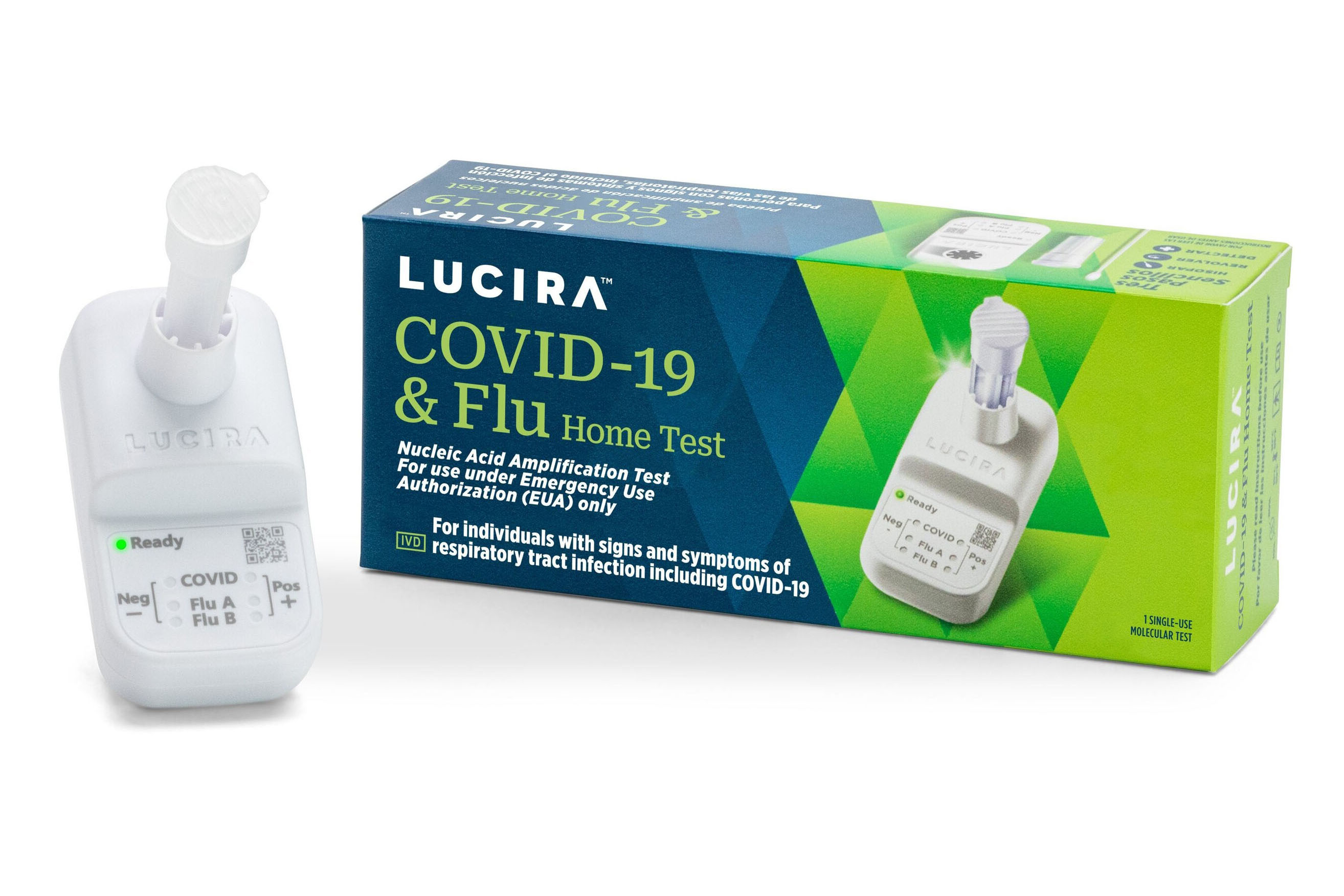
Project Overview
Our work with Lucira Health extends back to our days in Luke Lee’s lab at UC Berkeley, where we pioneered microfluidic technologies for point-of-care diagnostics. Lucira spun out of the university in 2013 with the mission of bringing laboratory quality diagnostics into the home and remote healthcare settings, empowering everyday people to make more informed health decisions.
The COVID-19 pandemic highlighted the critical need for accurate, accessible diagnostic testing outside traditional laboratory settings, and Lucira was uniquely positioned to address this need. The company received the first FDA authorization for an at-home COVID test in November 2020. We significantly scaled operations through 2021, culminating in an IPO that funded rapid manufacturing scale-up to 1M devices/month. The company went on to receive FDA authorization for the first over-the-counter combination flu and COVID-19 test just two days after declaring bankruptcy in February 2023, after a protracted regulatory review. Lucira became Mosaic’s first customer in 2021 and remained a customer through bankruptcy and subsequent acquition by Pfizer. The dramatic rise and fall of Lucira has been the subject of an academic case study.
Mosaic arose directly from the lessons learned at Lucira. We saw firsthand the immense challenges of translating university technologies into viable products. University research programs offer many things—insights into scientific and technology trends, mentorship to explore new ideas, and a network of lifelong colleagues and friends. But they don’t often prepare graduates for the realities of fundraising, product engineering, manufacturing, regulatory strategy, and commercialization planning. Over Lucira’s decade-long journey, we had to learn many things the hard way. We started Mosaic to help other academic innovators make fewer mistakes, make more efficient use of capital, and have a better shot at building successful products.
Technical Innovation
The Lucira product featured two main components: a sample vial and a microfluidic detection module. The device ran multiplexed LAMP (Loop-mediated Isothermal Amplification) with reverse transcriptase for detection of viral RNA targets for Influenza A, Influenza B, and SARS-CoV-2. A key benefit of these pathogens is that they can be easily lysed in a “single pot” reaction with detection reagents. We designed a compact microfluidic device consisting of three injection-molded components coupled to a low-cost electronic PCB heater/optical reader. The device could run up to eight independent LAMP reactions simultaneously. We designed the device to be fully self-contained and single-use, so cost optimizing the BOM and designing for assembly automation were of paramout importance. At scale, final retail price of the device was $35.
Key innovations included:
-
Colorimetric LAMP Assay: Novel detection method for isothermal nucleic acid amplification, dramatically simplifying readout instrumentation
-
Radial Microfluidic Design: Eight independent reaction chambers with integrated light pipes arranged in a circle ensured thermal uniformity and allowed multiplexed readout from a single photosensor.
-
Simple Electronic Readout: A single PCB provided assay incubation, detection, signal processing, and result display
-
Self-Sealing Vents: Innovative fluid loading mechanism allowing dead-end chambers to fill without valves
-
Battery Operation: Fully self-contained, powered by AA batteries
Overview of the Lucira device architecture and operation
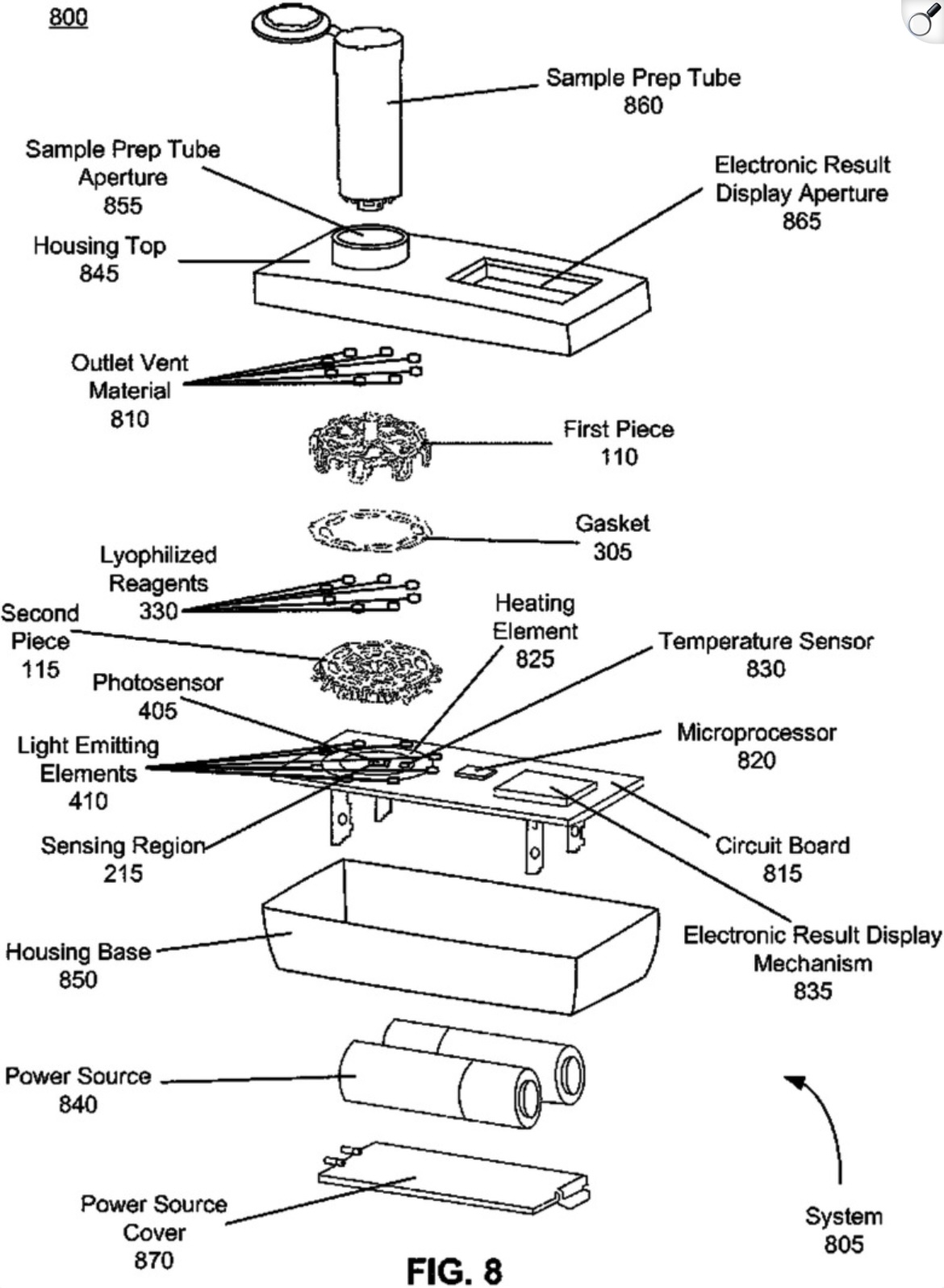
Lucira device architecture
Development Challenges
Lyophilized Reagents
The Lucira product featured a polymerase enzyme and other assay components which must be lyophilized for stability. Although there are many strategies for lyophilization, we chose pellet lyophilization because of its significant manufacturing scaling advantages. We worked closely with the original inventors of this technology, developing assay pellets which could be manufactured in bulk and reliably installed into the device. We built an in-house dry room with careful attention to electrostatic mitigation and developed robotic pick-and-place processes for pellet singulation, inspection, and placement. We worked closely with our lyophilization partners to optimize pellet integrity, execute accelerated aging studies, evaluate dessicant and packaging strategies, and define drying protocols for all device components.
Thermal Engineering
To achieve tight temperature tolerances across all eight reaction chambers, we optimized the PCB heater design through a combination of thermal finite element analysis (FEA) and experimental methods. The PCB included cutouts to isolate the heating area from the rest of the board, and we worked closely with our PCB vendors to ensure tight control of copper plating thickness.
Optical Engineering
The microfluidic module featured lensing elements and light pipes for collimnating LED light through the reaction chamber and down onto the surface of a central photosensor detector. We developed ray tracing simulations of this module, and we worked closely with our LED and photosensor vendors to define performance QC criteria.
Fluidic Engineering
As in any microfluidic system, bubbles and leaks a major concerns. This is especially true in the Lucira product, where a critical fluidic interface is made when the user attaches the sample vial to the detection cartridge. Any time you have user interaction with your product, expect variability. Any mating interfaces must be able to perform reliably in the face of this variability. We developed an innovative make-before-break interface using a minimal number of components which virtually eliminated the risk of bubble formation and leaks. We tested this extensively under different material conditions and attachment scenarios. We also developed on-device algorithms to detect and invalidate results when bubbles were present by analyzing telltale signs in the absorbance signal.
Supply Chain
Scaling a product to annual volumes exceeding 10M units/year during a global pandemic is an enormous challenge. We worked closely with our contract manufacturer as well as ever individual component vendor to meet our demand. For injection molded parts, this meant rapidly qualifying high-volume multi-cavity tooling, which presented many own challenges and required a number of follow-on tolerancing DOEs. For electronic parts, in a number of cases, there simply were not enough parts available in the market to support our demand. This meant designing and qualifying three alternative PCBAs, each running different firmware and a different configuration of parts.
Usability
Usability is one of the most critical design considerations of a consumer health product. We designed the Lucira product for simplicity. Through more than 1,000 observational user studies, we refined the design and instructional materials to ensure that anybody could use the product, sometimes evaluating the choice of a single word in the instructions. We measured tactile forces and developed design specifications to ensure our usability requirements would be met over the full range of material conditions.
Amplicon Containment
LAMP generates a significant amount of DNA amplicons, and we had to ensure that these amplicons did not escape the device and contaminate the environment (thus risking false positives on subsequent devices). We tested amplicon carryover extensively and we developed in-line manufacturing QC methods to monitor sealing efficacy of the self-sealing vents. As an added risk mitigation, a ziploc bag was provided with the product for secondary containment during disposal. Even with these measures in place, we had many issues with amplicon contamination at our facilities, and we developed rigorous operational protocols for monitoring amplicon contamination, mitigating personnel risk, and decontamination.
Design for Automation
We brought automation engineers into the design process very early on so that even before our first parts were injection molded, we had an automated assembly process in mind. The assembly process was developed with logical subassemblies which could be batch processed using automation-friendly processes and a minimal number of refixturing steps. We automated critical aspects of assembly during pilot production and ultimately the full assembly was automated as production scaled. We built functional PCBA test fixtures and the device also self-tested following assembly.
Impact & Timeline
- 2013: Founded as Diassess, Inc. by team members from UC Berkeley with SBIR funding
- 2015: Company raises seed round, joins Y Combinator and StartX accelerators
- 2017: Awarded $21M development contract from BARDA for a POC influenza test
- 2019: Initial FDA 510(k) application for flu test filed and later withdrawn
- 2020: Company becomes first to receive FDA Emergency Use Authorization for COVID-19 home test
- 2021: Successful IPO, scaled to 1M devices/month manufacturing capacity
- 2022: Health Canada authorization for combination flu & COVID-19 test
- 2023: FDA authorization for first OTC flu & COVID test (February 24); bankruptcy filing (February 22)
- 2023: Pfizer acquires assets & resumes production
- 2025: Pfizer ceases production
The platform demonstrated proof-of-concept for decentralized molecular diagnostics and established new regulatory pathways for consumer molecular testing.
Lessons That Built Mosaic
This journey taught us invaluable lessons that now form the core of Mosaic’s approach:
- Culture of Innovation: The most talented people want to work in a stimulating environment where new ideas are valued. Mosaic is selective with the projects and talent that we bring on. We focus on some of the most challenging technical problems. We actively encourage internal R&D, grant writing, and spin-outs.
- Experienced Advisors: Especially for areas such as commercial strategy, regulatory strategy, and manufacturing, it is critical to bring in advisors and consultants who can provide the expertise that only decades of experience can yield. We have a broad network of such advisors.
- Non-Dilutive Funding: Lucira was awarded nearly $30M in non-dilutive funding (grants and contracts) which was instrumental in getting the company off the ground and building a proof-of-concept with an investable thesis. At Mosaic, we’ve worked to streamline our process for SBIR grant writing. We contract with grant writers who have written thousands of SBIR proposals and we’ve built an LLM prompt pipeline to generate and evaluate proposals. We also sit on NSF and NIH SBIR review committees, where we’ve reviewed hundreds of proposals. We know what it takes to write winning grant proposals.
- Design for Manufacture: Understand the capabilities of the manufacturing processes you plan to use and build relationships with key vendors early. At Mosaic, we follow the latest trends on the manufacturing processes most common to biomedical products.
- Regulatory Strategy: For medical products (and some research products), it is critical to define a regulatory and reimbursement strategy early on. Mosaic partners with regulatory and reimbursement experts across various specialties within the biomedical space.
- Product-Market Fit: Technical excellence alone isn’t enough. Market timing, adoption curves, and competitive dynamics matter immensely. We can help you critically evaluate where your technology fits into the market landscape.
- In-House Prototyping: At Lucira, we worked with a combination of contract engineering resources and in-house resources. Ultimately, what we found was that having in-house resources was critical for driving a tight iteration cycle on prototyping and promoting tight integration across teams. At Mosaic, we’ve worked to bring all of the tools needed for building biomedical instruments under one roof, and we work closely with on-campus core facilities to augment our in-house capabilities.
- Vendor Network: We also recognize that we can’t do it all, and we shouldn’t try. That’s why we’ve built relationships with a number of key engineering and manufacturing vendors. If we feel that something is best done by a third party, we will say so.
- User-Centered Design: For consumer products, ease-of-use isn’t a feature, it’s a requirement. We bring the skills needed to conduct formative and summative user studies which guide product engineering.
- Capital Efficiency: We’ve built Mosaic from the ground up to be lean and capital efficient, utilizing AI throughout the organization. Additionally, we’ve built a network of consultants, freelancers, and vendors who can help us move projects forward without increasing square footage or headcount. We are experienced in modeling designs, and we know how to scope a prototype build to maximize learnings.
The experience of building Lucira from academic lab to IPO shapes everything we do at Mosaic. We help our clients navigate the complex path from innovation to market with eyes wide open to the challenges ahead.
Interested in developing breakthrough biomedical technologies? Contact us to explore how we can bring your vision to market with lessons learned from the front lines.